It’s hard to plan a trip when you don’t know exactly where you are going. You know you want to end up some place warm (who wouldn’t!), but you don’t know the exact highways or scenic detours to take to get there. You can’t be so rigid in your planning that you are unable to adapt to unforeseen changes along the way (road closures, traffic, etc,). However, if there isn’t any structure around your plan, you might never make it to your target location. Simply put, you need a map showing the many routes to get to your destination so you can plan as best you can before the trip is underway.
In the increasingly complex world of clinical trials, a Master Protocol is that map. The routes are created to evaluate more than one investigational drug and/or more than one tumor type. At each intersection the path forward is chosen based on data after the trial has begun.
Now instead of a traditional map leading to a physical destination, a simple way of visualizing master protocols studies are as baskets and umbrellas, or a combination of the two.
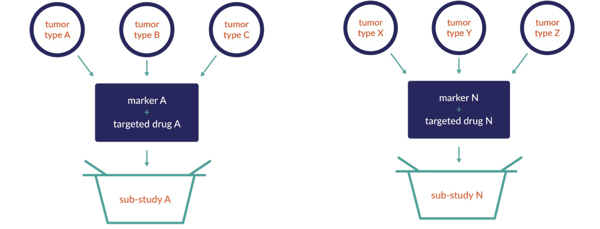
The basket trial involves a single investigational drug or drug combination that is studied across multiple cancer populations defined by disease stage or other marker. For example, all late-stage cancer patients will be treated by the same drug across several tumor types. Depending on the results, the path may lead to a sub-study allowing for expansion, and potentially expansion cohorts.
Basket Studies:
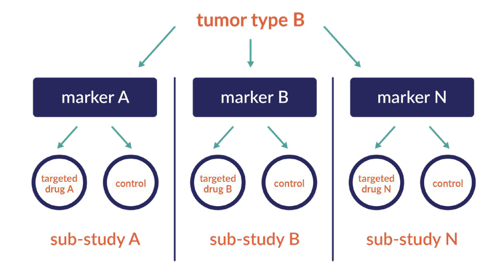
The umbrella trial is designed to evaluate multiple investigational drugs administered as single drugs or as combination drugs in a single disease population. For example, the umbrella is a broad disease sub-type (her2 positive breast cancer) with several treatment options under development. The path may lead to dose finding cohorts to identify safe doses for the most effective treatments.
Umbrella Studies:
Master protocols may even combine the two, for example by starting with a dose escalation using multiple combination therapies (more of an umbrella) accepting any type of solid tumor and then refining their recommended phase two dose in more specific tumor types (more like a basket).
If your mind is spinning with pictures of baskets, umbrellas and all the possible paths, we are right there with you. The common denominator is that the sponsor does not know what will be effective. The key is to follow positive signals and refine the treatment as data is collected over the course of the study.
Easier said than done? Sure. Are there many operational challenges along the way? Of course. However, technology does not have to be one of them.
Master protocols are critically dependent on configurable and flexible randomization and trial supply management (RTSM) systems. Not only can you build in flexibility from the onset, but mid-study changes and amendments can become faster and more efficient while offering robust quality.
While you may not know exactly where you are going, you can have confidence that you can follow the science of the trial.
To learn more, download our recent webinar recording:
Tag(s):
Study Execution
