RTSM Solutions for
Phase 1 Trials
Prancer RTSMⓇ enables you to follow the science. Whether you have a simple, first-in-human study or complex dose escalation trials, our RTSM solutions provide the flexibility to adapt and scale alongside your Phase 1 trials.
From Simple First-in-Human
to Complex Oncology Trials
We understand that Phase 1 trials vary in complexity. This is why Prancer RTSMⓇ can be configured to provide the features you need, when you need them. Discover how we can support both simple and complex trials by exploring our solutions below.
Prancer RTSM® Lite
Ideal for simple, first-in-human studies
- Track inventory and patient activity
- Assign treatment arms using randomization or cohort assignments
- Fixed dispensing logic
- Essential functionality only
Prancer RTSM®
Ideal for complex dose escalation and expansion studies
- Extensive cohort management functionality
- Any randomization types, including minimization
- Fully customizable dispensing logic
- Robust, advanced features and functionality
 Phase 1 trials can be even more complex than their Phase 2 and 3 counterparts. Change is an inevitable part of the trial design process, and in some cases protocols are undergoing revision as soon as the initial protocol version is approved.
Phase 1 trials can be even more complex than their Phase 2 and 3 counterparts. Change is an inevitable part of the trial design process, and in some cases protocols are undergoing revision as soon as the initial protocol version is approved.
Jonathan Pritchard, Commercial Operations Manager, 4G Clinical
Solutions that adapt to meet your unique trial needs.
Our randomization and trial supply management solutions help you navigate the unpredictability of Phase 1 clinical trials.
-
First-In-Human Studies
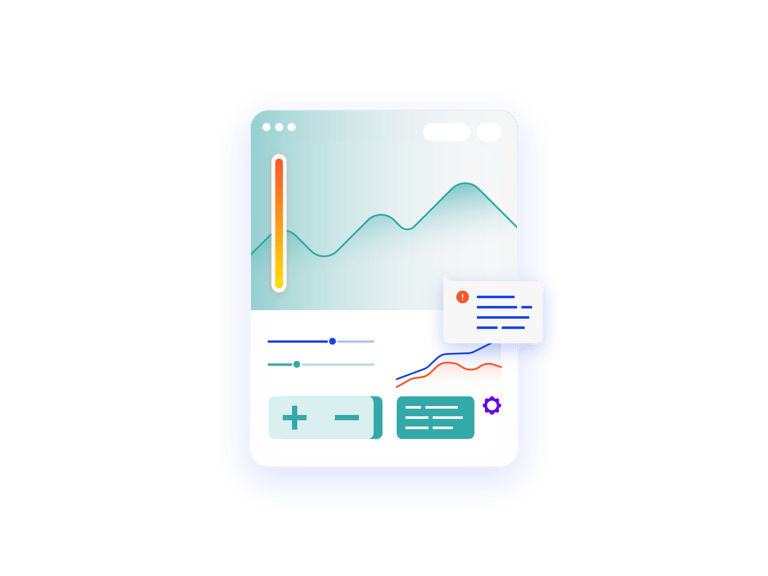
Inform strategic decisions for Phase 1 trials.
Prancer RTSMⓇ Lite provides visibility into trial data so you can help inform strategic decisions regarding your Phase 1 trial. Transitioning from manual management of first-in-human trials also reduces human error and improves accuracy.
Learn MoreEasily expand to a more complex study design.
Once you receive promising results, you may want to quickly change to a more complex design. While most IRTs cannot scale with your study, Prancer RTSMⓇ Lite can.
-
Dose Escalation and Expansion Studies
Learn More
Enable adaptive trial designs for evolving studies.
Prancer RTSMⓇ allows you to build in blank cohorts within the design that you can assign as needed with our self-service cohort management functionality.
 Learn More
Learn MoreAdvanced features support even the most complex trial designs.
Prancer RTSMⓇ offers protocol versioning on a per-site and per-cohort level, per-site reservations to guarantee a slot in a cohort, and a robust audit trail traced to each protocol version.

Inform strategic decisions for Phase 1 trials.
Prancer RTSMⓇ Lite provides visibility into trial data so you can help inform strategic decisions regarding your Phase 1 trial. Transitioning from manual management of first-in-human trials also reduces human error and improves accuracy.
Easily expand to a more complex study design.
Once you receive promising results, you may want to quickly change to a more complex design. While most IRTs cannot scale with your study, Prancer RTSMⓇ Lite can.

Enable adaptive trial designs for evolving studies.
Prancer RTSMⓇ allows you to build in blank cohorts within the design that you can assign as needed with our self-service cohort management functionality.


Advanced features support even the most complex trial designs.
Prancer RTSMⓇ offers protocol versioning on a per-site and per-cohort level, per-site reservations to guarantee a slot in a cohort, and a robust audit trail traced to each protocol version.
Scenario Modeling for Supply Planning
Assumptions shift. It is important to have a conversation with your clinical operations counterparts on the impact of trial changes. This is why scenario modeling is so valuable.
RTSM Solutions for Any Trial Complexity
Our randomization and trial supply solutions can easily be configured to include only the needed functionality and are built to scale with you as your clinical program progresses.
Prancer RTSM®
100% configurable and flexible solution with robust features and functionality to meet the needs of any trial.
Learn MoreAll Products
Explore our full suite of solutions for randomization and trial supply management (RTSM) as well as clinical supply optimization and clinical forecasting.
Learn More