The Science Leads. We Guide.
As your critical partner, our thought leaders and clinical supply experts can help guide you in everything from RTSM and supply decisions to how emerging industry trends may impact your clinical trials. We’ve been listening to industry experts and site users from the beginning, to build our industry-leading, highly configurable RTSM system.
We’re an extension of your trial team.
Your trusted partner throughout the trial, from initial design meetings to protocol amendments to study conclusion.
-
Our People
Meet Our Team
Our experts that guide your study.
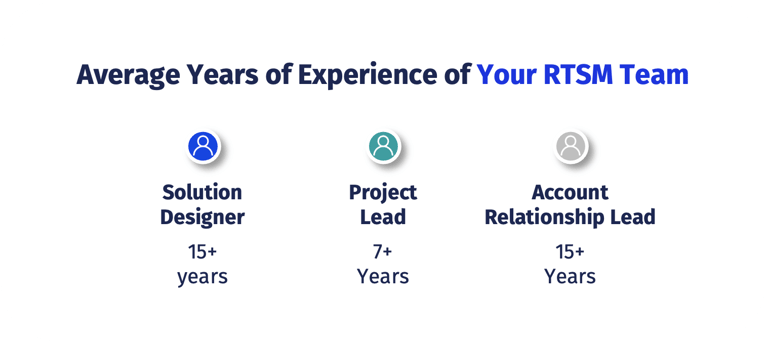
4G Clinical’s team brings hundreds of years of cumulative experience in clinical trial development to the table, across all therapeutic areas and indications, always keeping the patient at the forefront of our work.
 See How Our Solutions Enable Complex Design
See How Our Solutions Enable Complex DesignUtilizing our experience to enable complex protocols.
As today’s complex trials evolve, you need a team that knows how to adapt to change, and how to update the study for optimal outcomes.
-
Our Process
Learn More about our agile RTSM development
Leveraging an agile RTSM methodology to accelerate study start-up.
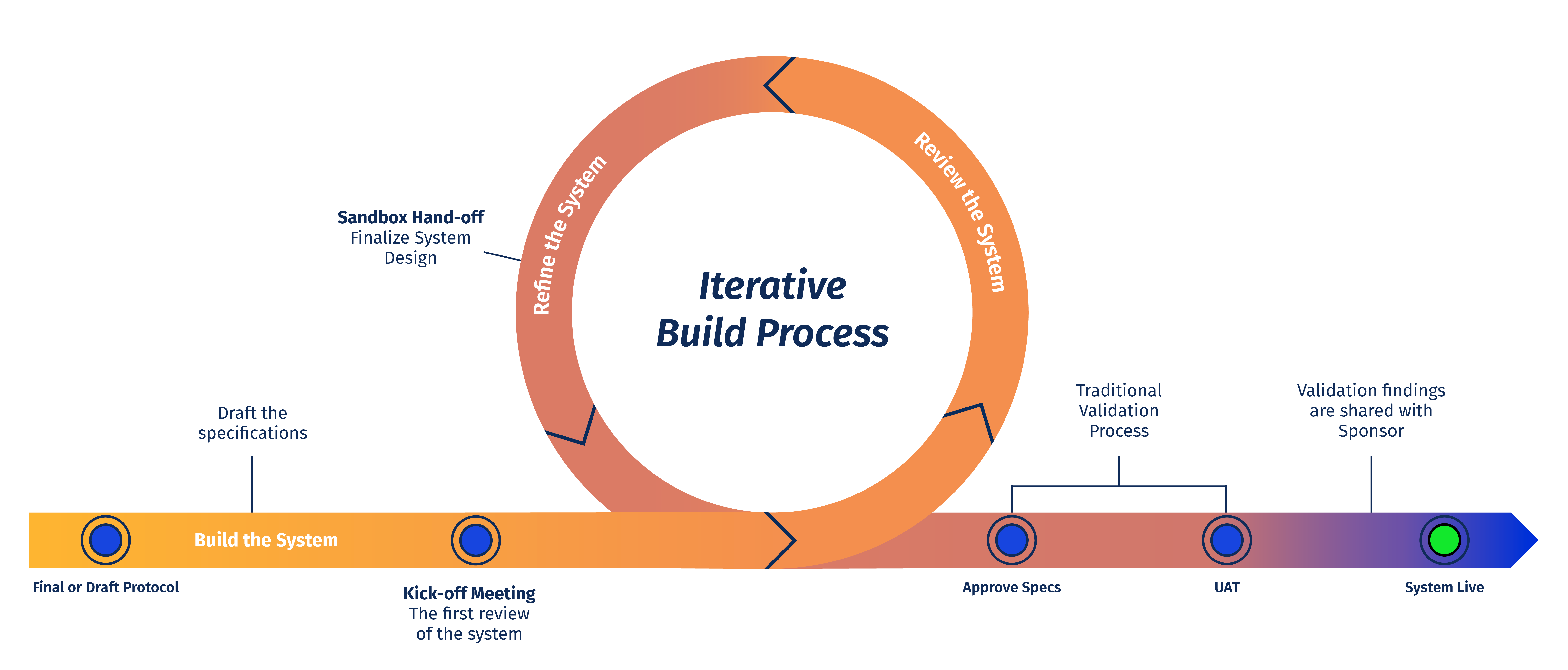
The benefit to the customer of a truly agile RTSM methodology is the ability to interact with the system and provide feedback during several iterative process loops. They know what system they are getting way before they sign- off on the specifications. The increased transparency and customer involvement from receipt of the draft protocol ensures UAT will be a final check and there will not be delays to their study.
-
Our Product
Explore our solutions
Solutions built from industry expertise.
Our Co-Founder, Ed Tourtellotte, has built RTSM software before. Twice. But, with the ever-changing industry landscape of complex protocols and supply requirements, how can we build the 4th generation of RTSM that is truly agile, flexible and easy-to-use? Hence, the inception of 4G Clinical.

Our experts that guide your study.
4G Clinical’s team brings hundreds of years of cumulative experience in clinical trial development to the table, across all therapeutic areas and indications, always keeping the patient at the forefront of our work.


Utilizing our experience to enable complex protocols.
As today’s complex trials evolve, you need a team that knows how to adapt to change, and how to update the study for optimal outcomes.
Leveraging an agile RTSM methodology to accelerate study start-up.
The benefit to the customer of a truly agile RTSM methodology is the ability to interact with the system and provide feedback during several iterative process loops. They know what system they are getting way before they sign- off on the specifications. The increased transparency and customer involvement from receipt of the draft protocol ensures UAT will be a final check and there will not be delays to their study.

Solutions built from industry expertise.
Our Co-Founder, Ed Tourtellotte, has built RTSM software before. Twice. But, with the ever-changing industry landscape of complex protocols and supply requirements, how can we build the 4th generation of RTSM that is truly agile, flexible and easy-to-use? Hence, the inception of 4G Clinical.

Expertise That Transcends Borders and Boundaries
4G Clinical’s thought leaders and clinical study experts are with you throughout, providing sound advice for every aspect of your study, no matter where the science leads, or where your patients reside.
-
Global Reach
We benefit patients on a worldwide stage.
We are a global company, with a universal goal of helping patients. With operations teams in nine time zones across the globe, we aim to accurately represent the international nature of this industry, and of the populations we serve.
Learn More -
Act Locally
Support your global trials with a local touch.
Configure country-specific patient flows and support diversity in trial participation with advanced cohort capabilities and adaptive trial designs. Set resupply parameters globally all the way down to individual sites and support access to trials with decentralized or personalized distribution models.
Learn More -
Thought Leadership
Our people help move the industry forward.
4G Clinical has recruited clinical trial experts from all corners of the international biopharma industry. Currently, our leaders hold positions on prominent boards, and are active in industry committees, offering their expertise well beyond our walls.
Learn More
We benefit patients on a worldwide stage.
We are a global company, with a universal goal of helping patients. With operations teams in nine time zones across the globe, we aim to accurately represent the international nature of this industry, and of the populations we serve.
Learn MoreSupport your global trials with a local touch.
Configure country-specific patient flows and support diversity in trial participation with advanced cohort capabilities and adaptive trial designs. Set resupply parameters globally all the way down to individual sites and support access to trials with decentralized or personalized distribution models.
Learn MoreOur people help move the industry forward.
4G Clinical has recruited clinical trial experts from all corners of the international biopharma industry. Currently, our leaders hold positions on prominent boards, and are active in industry committees, offering their expertise well beyond our walls.
Learn More