July 17, 2019
Top 6 Benefits of Leveraging an RTSM for Investigator Initiated Trials (IITs)
Written by: 4G Clinical
Investigator Initiated Trials (IITs), sometimes called Investigator Sponsored Trials (ISTs), are executed under the direction of third-party clinical investigators who are physician researchers, often within an academic institution. However, it is typically the Medical Affairs Department, not Clinical Operations or Drug Supply, that approves the protocol and requests for drug.
This presents challenges with supply chain management since IITs traditionally draw from the same pool of drugs as manufacturer-sponsored studies. Project too much, you waste expensive drug. Project too little, you risk stock-out. With 100s of IITs at any given time, the manual process of tracking drug is not sustainable. There also lacks visibility between Medical Affairs and their Clinical Operations and Drug Supply counterparts on expected demand.
The solution? A modern, flexible randomization and trial supply management (RTSM) can alleviate these challenges. Here are the key benefits:
1. Avoid Wasted Supply
Some sites ask for several years of drug supply, without taking into consideration expiry. This results in wasted drug. What if sites could order drug directly from the RTSM? Using the RTSM capability for capturing site orders, sites can request the quantity of inventory they need a month or two at a time. In doing so, there could be justification requirements for the quantity needed rather than accepting all requests without context. The site may be required to enter the number of patients this will supply, desired arrival date, how many more patients are anticipated, anything the manufacturer needs to know that would help manage supply.
2. Ensure Visibility into IIT Supply Demand
What if demand can be entered by simply adding patients and using the RTSM to perform very simple dispensing? Or, have sites periodically mark their current inventory as consumed. Then, an integrated site forecasting functionality can be used to project patient demand or check for minimum inventory levels and automatically resupply. It can be that simple.
3. Accurately Project Demand for both IITs and Manufacturer-Sponsored Studies
Another key benefit of using an RTSM is the ability to easily project future visits and upcoming demand across all studies, regardless of who is sponsoring them. This helps the Clinical Operations and Drug Supply groups manage supplies for all Phase 1-3 studies, to ensure they have enough to cover both groups of studies and minimize waste.
4. Maintain Record of IIT Shipments to Inform Sunshine Reporting
Manufacturers also must track drug given to the sites for sunshine reporting. The PIs are not paying the manufacturer for the drug, but rather have an arrangement to share data and report findings together. Therefore, the value of the drug given to the PI becomes subject to sunshine reporting requirements. The RTSM maintains a record of shipments to facilitate sunshine reporting. No more manual tracking and reporting.
5. Alleviate the Burden of Manual IST Tracking
Manual management of the IIT supply is not scalable, so with an increasing number of IITs, automation of this process is critical. There are too many moving parts, drug is too expensive to waste and too important to not have enough.
6. Pool Supply to Reduce Waste
Through all of these options, the RTSM would allow the manufacturer to pool supply across shipping locations, achieving supply efficiencies. Mix and match different solutions to fit each drug program and each site’s needs but keep a single pool of supply that can flex to go anywhere.
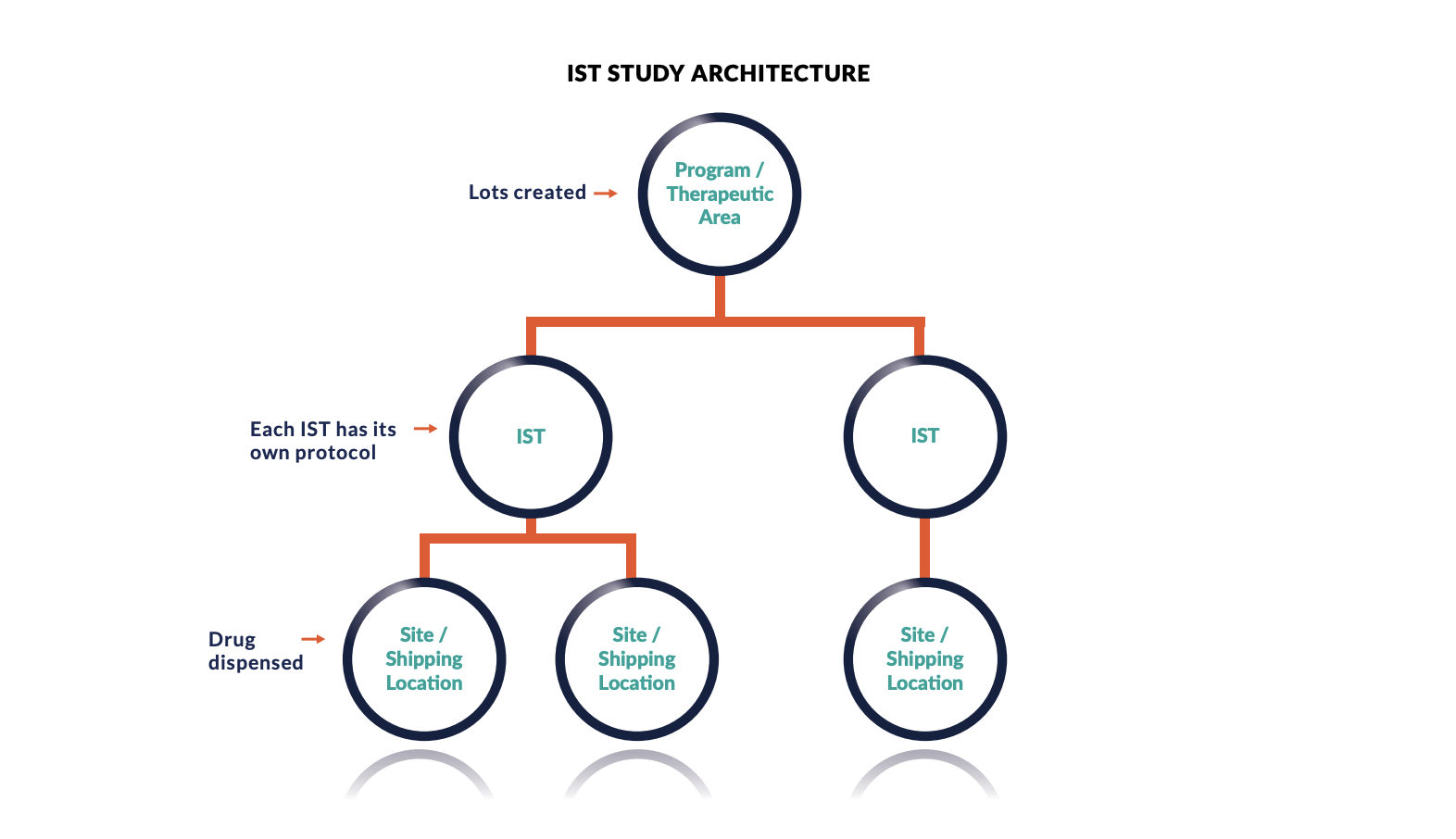
The one caveat – Your RTSM needs to be configured (and flexible enough!) to adapt to the different terminology and architecture required for an IIT.
Tag(s):
Study Execution
