December 7, 2023
Safeguarding Success: Outlining the Crucial Role of Experience in Patient Safety and Trial Success
Written by: Elizabeth Rickenbacher
Within the world of clinical trials, the intersection of experience and innovation is instrumental in ensuring patient safety and the overall trial success. In this blog, we explore key aspects that underscore the indispensable role of experience in RTSM design to ensure the implementation of the patient visit and dosing schedules match protocol design and study team expectations.
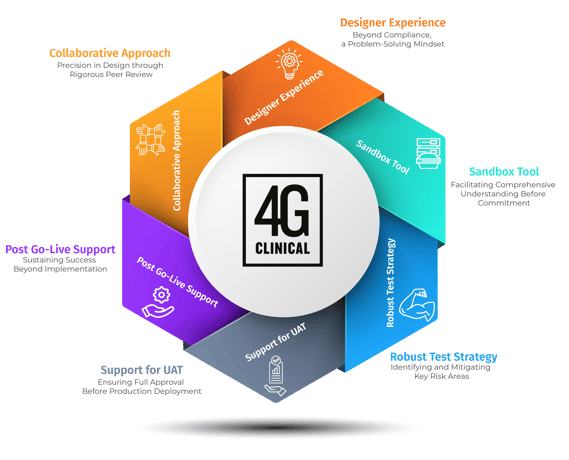
Collaborative Approach: Precision in Design through Rigorous Peer Review
Partnership and collaboration are fundamental to the successful translation between clinical trial protocol and the design of the RTSM system in use at clinical sites. Open communication between the study team and the RTSM partner allows for a design approach and review process which supports an iterative design process so that gaps in expectations/requirements can be identified and properly addressed. In the design phase, peer review plays a pivotal role, subjecting trial designs to scrutiny from diverse perspectives allowing the technology vendor to provide best practice solutions based on experience and collaborative efforts. This comprehensive assessment establishes a robust foundation, fostering innovation, identifying potential blind spots, and instilling confidence in the final design. This collaboration does not only yield a final product design, but also a foundation to partnership which supports the trial for the duration of the study. Decisions made together will ensure that the sponsor, sites, and vendor remain aligned long after the first patient is enrolled.
Designer Experience: Beyond Compliance, a Problem-Solving Mindset
Every trial requires nuanced consideration and alignment on how to implement the study design within the RTSM system should never feel transactional. Collaboration with the problem-solving mindset of an experienced designer building the RTSM system is perhaps the most important differentiator which should be considered prior to choosing your technology partner. Accomplished system designers who understand protocol design, trial/therapeutic requirements, regulatory requirements, and the RTSM technology are what distinguish the ease at which study teams can navigate the demands of complex trial design implementation into the technology systems relied upon at clinical sites. Supporting vendor experience with Sponsor subject matter experts (e.g. supply manager and clinical operations team) allows for a robust review of study requirements to ensure all aspects of the trial design are met prior to implementation. Rather than viewing protocols as rigid templates, experience provides a different approach, balancing the appropriate factors into systems which are user friendly and trusted by sites to guide their patients through life changing clinical trials.
Sandbox Tool: Facilitating Comprehensive Understanding Before Commitment
As mentioned, each trial has their own unique considerations, and as such, a comprehensive understanding of the RTSM system’s features and end user options should have the opportunity to be scrutinized beyond the specification documentation only. Ensuring alignment among stakeholders before advancing to subsequent validation phases is a common challenge in RTSM system design and adoption. Supporting Sponsor understanding of the RTSM system and the implemented design by utilizing a study specific sandbox environment, rather than solely relying on specification documents, provides the Sponsor with a comprehensive and interactive view of the trial design to ensure all requirements are met. This tool serves as a bridge, translating complex trial specifications into a tangible and interactive format. Stakeholders engage in hands-on exploration, addressing ambiguities and achieving consensus before finalizing the trial design giving them assurances the design not only meets protocol requirements but also site useability requirements.
Robust Test Strategy: Identifying and Mitigating Key Risk Areas
Once the trial design has been finalized through the above described collaborative and hands-on approach, only then should the RTSM design be rigorously assessed against expectations and tested. Experience-driven clinical trial design prioritizes the development of a robust test strategy. This comprehensive plan for internal testing aims to identify and address key risk areas, minimizing potential disruptions during the actual trial. Referencing not only the design specification, but the protocol and lead designers input on the configuration allows for a comprehensive validation strategy to be established. A seasoned team, drawing from experience, anticipates challenges and strategically tests the trial design in a controlled environment. This proactive approach enhances the overall resilience of the trial design and contributes to the seamless execution of the study.
Support for UAT: Ensuring Full Approval Before Production Deployment
Once the trial design has been implemented and vigorously tested against design requirements, the sponsor should evaluate the decisions made and how the RTSM supports their trial expectations as they perform user acceptance testing (UAT). Experience-driven RTSM design ensures that the system is not only technically functional but also aligns seamlessly with the needs and expectations of the study team. Supporting UAT with the previously used sandbox, provides a starting platform for UAT as the Sponsor has the opportunity to become familiar with the user roles and overall functionality of the system, allowing for a more robust and directed UAT experience. Additionally, offering active support to the sponsor and assessors during UAT allows experienced designers to collaborate to address concerns and suggestions enhancing the effectiveness of the UAT process. This ensures not only technical approval but also enthusiastic endorsement by end-users, facilitating a smooth transition to the production phase.
Post Go-Live Support: Sustaining Success Beyond Implementation
In the post go-live phase of a clinical trial, ongoing support plays a pivotal role in sustaining success beyond implementation. Effective maintenance phase oversight should be made possible through reporting, alerts, and notifications which can be leveraged to meet the individual needs of the end user and their operating area. Utilizing your technology vendor as an extension of your study team encourages collaboration, enabling teams to highlight areas of risk to ensure solutions are provided to prevent issues rather than being reactive to them. Continuous and open communication establishes a partnership for the overall success of the trial. In addition to supporting ongoing maintenance needs, the importance of comprehensive study change and amendment support should also be considered as a determining factor when choosing an RTSM vendor. Planning for system changes in advance can support the fluidity of amendments where possible, however, by utilizing RTSM expertise, the original design should allow for unexpected changes to be implemented where needed. Diving into the purpose of a sponsor’s request allows for effective implementation and ensures that the change requested meets the true goal of the sponsor. Post go-live support goes beyond troubleshooting; it fosters a culture of continuous improvement, with insights from monitoring and assessments, informing strategic adaptations for future challenges. This holistic approach, guided by experience, ensures that each clinical trial remains not just a study, but a beacon of innovation and patient-centric care.
Conclusion
In the intricate realm of clinical trials, the fusion of experience and innovation is non-negotiable. A collaborative approach, coupled with a problem-solving mindset, underscores the importance of diverse perspectives in RTSM design. Utilizing tools like the sandbox environment and implementing robust testing strategies further solidifies the foundation of success. Experienced RTSM system designers, functioning beyond mere box-checking, bring forth a nuanced understanding of trial dynamics, ensuring adaptability within the framework of regulatory guidelines. As we navigate the future of clinical trials, the role of experience in safeguarding success becomes increasingly evident. It transcends the mere act of designing technology systems to support trials; it entails orchestrating a symphony of expertise, collaboration, and foresight to ensure the well-being of patients and success of the clinical trial.
Elizabeth Rickenbacher
Libbi Rickenbacher, Sr. Director of Product Strategy and Partnerships, has over 15 years of experience in the field of life sciences and is an e-clinical RTSM solutions subject matter expert. Her experience lies in building and managing accounts, clients, and strategic initiatives.
