March 18, 2020
A Lung Cancer Patient & Clinical Trial Participant Weighs in on COVID-19
Written by: 4G Clinical
4G Clinical's VP of Marketing, Amy Ripston, had the unique opportunity to interview Linnea Olson, a lung cancer patient and all around amazing human being, on the impact COVID-19 has had on her life.
Since the original posting of this blog, Linnea unfortunately passed away after a long courageous battle. She was an inspiration to so many and we are honored to have known her. Please read. Please share. Her voice, and the voice of patients, needs to be heard.
This is not a typical post for me, though we are finding ourselves in unprecedented times. So here we go. It is real. It is raw. It is human.
We are now facing a crisis. COVID-19 has emerged as a global pandemic and we’ve seen the impacts on everyday existence, local communities, economics. Infections know no boundaries. Social distancing has become the new normal. You are working from home. You may also be home schooling your children. You are limiting contact with family and friends.
In the midst of this, there are a myriad of biopharmaceutical companies developing vaccines as swiftly as possible. Hope of a treatment is there, albeit farther away than is desirable. With all the media attention towards the Coronarvirus, let’s not forget that clinical research must go on. There is still no cure for cancer, and there are countless immunocompromised clinical trial participants sitting at a precipice of fear, uncertainty and hope.
Meet Linnea Olson. 15-year lung cancer survivor. 4-time clinical trial participant. Patient advocate and activist. Parent. One amazing human being.
Last week, Linnea went to Massachusetts General Hospital (MGH) for a lung biopsy that was part of her clinical trial. She was nervous to go to the clinic since MGH is one of the main hospitals where positive COVID-19 patients are being treated. She is very high risk. She is also required per her study protocol to have tests, scans, etc. to remain enrolled. Enrolled in a trial that is keeping her alive.
Let that sink in for a minute. The biopsy was to provide data to the pharmaceutical company for the clinical trial. The data was not going to be shared with her physician so it could be used to aid in her care. On top of visits for the clinical trial, she also has visits that are necessary for her health like her infusions, which can only be done in a hospital setting. It is a risk either way. Don’t go to the hospital and you are unable to receive life-saving infusions and treatment as part of a clinical trial, do go and you expose yourself to catching COVID-19.
If put in this position, what would you choose?
Linnea’s personal mantra is “In every crisis, there is opportunity. A way to do things better.” I sat there and listened to the mix of hope and despair in her voice. She has been fighting for survival for the past 15 years. She’s seen her children become adults, something she never thought would happen. She is grateful. She is fearful all her struggle will come down to a battle with a virus. She is also exhausted and ready for the industry to change.
Now is the time to take a step back and ask some tough questions, and find the best path forward. Here are just a few questions I discussed with Linnea; Each one deserves its time of discussion.
1. Should the industry move towards Direct-to-Patient (DtP) or Decentralized Clinical Trials?
Patient safety is paramount. If there is a way to limit possible exposure by having some of the visits done at home, it is a win for patients. For Linnea, her current clinical trial and healthcare requirements do not have the infrastructure to support DtP. There are very strict requirements on where/how her treatments can be administered. This would require a protocol amendment to add in flexibility, as well as the hospital itself extending its services outside of the main clinic. Not out of the realm of possibility, but requires all stakeholders to embrace a new approach. Candidly, Linnea was also not keen on the idea of DtP in the past. She liked the idea of increased accessibility but wondered what it would be like to remove human touch. The current healthcare crisis has been a lesson for everyone, as she feels human touch now seems more of a luxury and a liability. She still has reservations about welcoming others to her home as it is the only place she considers a safe-haven. Bringing in others can also increase risk – less risk, however, than a clinical setting.
2. Are chronically ill patients dispensable?
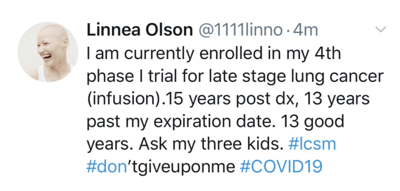
To be honest, this one hurt to listen to. Put yourself in Linnea’s shoes. She feels she has been a burden on the current healthcare system for years. As we are headed to a place where non-essential services are cancelled, she feels very vulnerable. Will her treatment be considered non-essential? Will the trial shut down?
This is having major impacts on the morale of patients. That she somehow has to prove that she is worth saving, that she and others like her would be in the “last to treat” category. It really is devastating.
Your initial reaction may be – Of course not! But then you look at the dire situation coming out of Italy where decisions are being made on who lives and dies as in war. Chronically ill patients and elderly may not fare well. While we all hope this will not be the case, it is certainly a growing concern for trial patients.
3. Can current trials be amended to only include visits that are truly necessary, or altered to ensure the patient benefits?
In this current environment, it should be considered to go back through and ensure each visit, each test, each element of data collection is absolutely necessary to the success of the trial as well as its impact on the health and well-being of trial participants. Are data points being collected to just have more data or is it critically necessary? If it is necessary, trial participants would love to understand why. To not feel like part of a science experiment, but rather a partnership. A little can go a long way.
Like Linnea, I didn’t fully understand the distinction between a research and a clinical biopsy. For the research biopsy, the data would not even come back to her own physician. Even when that data could help inform her care and others in her same situation. The question becomes not only is it essential, but is there a way to share results?
I could have talked to Linnea for hours, but I wanted to leave this post with a few calls for action.
What if the industry reacted to this crisis by making some positive changes? Trials could start moving to a more decentralized approach to prevent immunocompromised individuals from traveling to clinical trial sites for visits that can be performed at home. Protocols can be amended to remove any non-essential testing that can’t be done remotely that is not 100% necessary in the current environment.
What if patients start asking more questions about their visits, on the use of their data, on the trials themselves? If enrollment and retention start becoming even more challenging, will that be the catalyst to start a Q&A at the onset? Will patients be more likely to risk going to the clinic and stay in the trials if they understand what the data will be used for, and perhaps, also be able to share it with their physicians in the hopes of improving their own health?
I encourage each of you to read Linnea’s blog: https://outlivinglungcancer.com/ and to listen to her recent Ted Talk: https://tedxbeaconstreet.com/videos/patient-parent-person-research-subject/.
Tag(s):
Industry Voices
