Advanced Clinical Trial Supply Optimization
4C SupplyⓇ addresses the increased pressures on clinical supply professionals to minimize waste and avoid stock-outs as clinical trial complexity continues to rise.

Surface supply chain risk.
4C SupplyⓇ empowers supply chain managers to surface and mitigate risks before they happen. By calculating the resupply floor, our powerful clinical supply forecasting solution dynamically adapts to unexpected shifts in enrollment and impacts from protocol amendments.
Create supply chain visibility.
Explore how 4C SupplyⓇ enables you to make data-driven decisions through powerful scenario modeling and visualizations impacting your clinical supply, clinical operations and manufacturing organizations.
-
Shipment Size
and FrequencyIdentify the proper quantity of drug to ship over time.
4C SupplyⓇ assists you to balance the cost of frequent shipments against the cost of wasted inventory at your sites.

-
Risk-Based Supply
Decision-MakingUnderstand the impact of changes on your clinical trial.
4C SupplyⓇ provides total study cost reports so you can weigh increases in enrollment against added costs from study changes such as adding new countries. This allows you to make the best decision for your trial with full visibility of its impact.

-
Supply Chain Agility
Dynamically adapt as your clinical trial evolves.
Model ‘what if’ scenarios for dose escalation and dose finding studies. 4C SupplyⓇ provides visibility into the supply impacts involved with cohort management in complex studies.

-
Aggregate Demand Planning
Ensure capacity for manufacturing by predicting aggregate demand.
4C SupplyⓇ pulls in data and can show you an aggregated demand view that is based on the same mathematical assumptions. Data from 4C SupplyⓇ can be pushed into an ERP and used to roll up numbers so CMC knows how much API needs to be produced to meet study numbers.
Identify the proper quantity of drug to ship over time.
4C SupplyⓇ assists you to balance the cost of frequent shipments against the cost of wasted inventory at your sites.

Understand the impact of changes on your clinical trial.
4C SupplyⓇ provides total study cost reports so you can weigh increases in enrollment against added costs from study changes such as adding new countries. This allows you to make the best decision for your trial with full visibility of its impact.

Dynamically adapt as your clinical trial evolves.
Model ‘what if’ scenarios for dose escalation and dose finding studies. 4C SupplyⓇ provides visibility into the supply impacts involved with cohort management in complex studies.

Ensure capacity for manufacturing by predicting aggregate demand.
4C SupplyⓇ pulls in data and can show you an aggregated demand view that is based on the same mathematical assumptions. Data from 4C SupplyⓇ can be pushed into an ERP and used to roll up numbers so CMC knows how much API needs to be produced to meet study numbers.

Empower business-critical decisions with powerful visualizations.
4C SupplyⓇ provides dynamic reporting to assist with internal communication and strategic decisions impacting clinical trial programs.
-
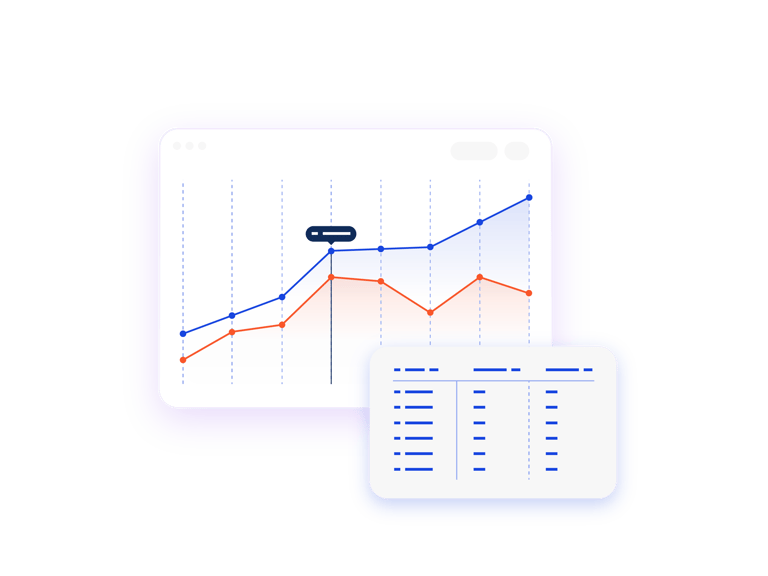
Compare “What If?” Scenarios
The scenario comparison report enables you to compare two or more finished scenarios with or without IRT actuals. Use this information to compare monthly forecasts and provide feedback to Clinical Operations on potential changes to the protocol.
-
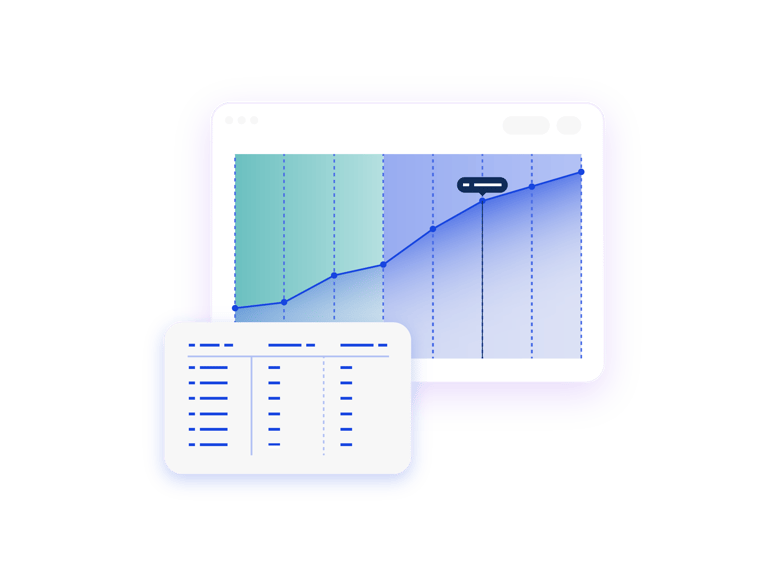
Understand Total Drug Dispensed
The Unconstrained Patient Net Demand report shows you the total patient consumption for your study. When paired with IRT actuals, this report shows you what has been dispensed to date vs. what is still forecasted to be dispensed.
-
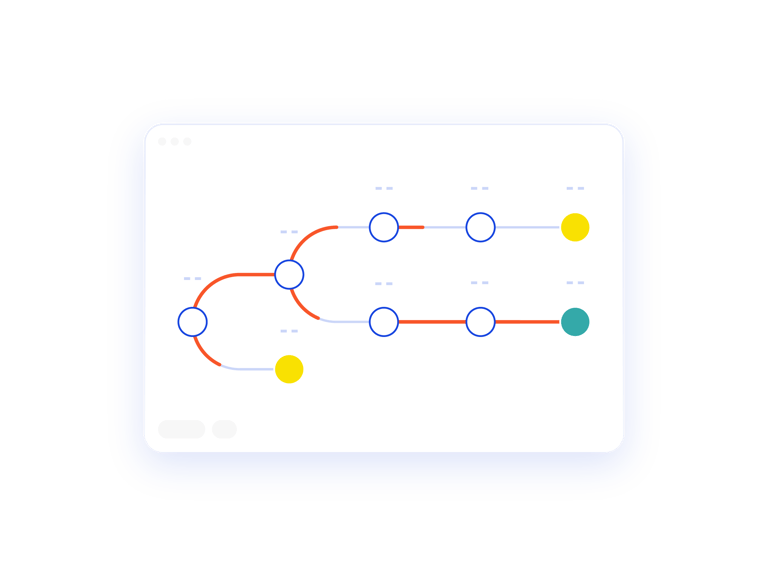
Map the Patient Journey
The Patient Treatment Tree is a visual representation of the patient journey. This report allows you to verify your inputs against expected outcomes.
-
View Production Demand in Aggregate
The Manufacturing Aggregation report allows you to aggregate two or more study kit production demands into one report to visualize what the production needs for the duration of each trial will be. This report is ideal for studies that share identical kit types.




 4C SupplyⓇ provides the ability to build out a supply model on a single screen and enables you to input data in any order or sequence. Along with its simplicity, 4C SupplyⓇ can easily be updated with additional information as it becomes available.
4C SupplyⓇ provides the ability to build out a supply model on a single screen and enables you to input data in any order or sequence. Along with its simplicity, 4C SupplyⓇ can easily be updated with additional information as it becomes available.
Laurel Ferenchick, Senior Forecasting Services Lead, 4G Clinical

Gain simplicity and control over clinical supply management.
You no longer have to worry about information getting lost or going out of sequence when using spreadsheets or other clinical supply optimization software. Data is entered into 4C SupplyⓇ in text format, similar to a study protocol outline, in any order or sequence to build out a study model on a single screen.
A supply solution that scales with your trials.
Instead of forcing you to define all countries or depots upfront, 4C SupplyⓇ can easily be updated with additional detail as it becomes available rather than force-fitting unknown inputs. Whether you are starting out with little information or you are incorporating IRT actuals, 4C SupplyⓇ adapts to any level of information and scales to meet your trial needs.
Integrate with any IRT.
When a study is live, actuals data from Prancer RTSMⓇ or any clinical IRT solution can be fed into 4C SupplyⓇ. Supply managers can compare study assumptions against the actuals and potentially uncover unexpected shifts in enrollment and initiate conversations with key stakeholders.
